Earth Part 4/4 - Life on earth, unique in the universe?
The key question: “Are we alone in the universe?” still deserves an answer in my lifetime. I feel that a ballast would fall off my shoulders. To ease the pain, here is an overview.
Exoplanets.
An exoplanet is a planet orbiting a different star from our sun. The first discovery dates back to 1995, the Swiss astronomers have received the Nobel Prize. They used little dips in the light that the other star was emitating every time a planet passed by her. So we are talking about indirect observations that allow us to make all kinds of calculations to conclude that these eclipses are due to a passing planet.
If such an exoplanet meets certain conditions, it is ranked among the potentially habitable planets. For example, the size of that planet and the distance from its parent star play an important role. The hunt for habitable planets has been greatly stepped up in recent years and several thousand are already known.
Nevertheless, there is very little chance that we will ever take one step there. The biggest obstacle is the enormous distance that is several decades light years.
Water.
Life as we know it requires a lot more than just water, but water is necessary.
Biosphere 2 ended in a fiasco in 1991. The oxygen levels dropped dramatically and the inhabitants were constantly hungry, as many plants died of water shortages and a deficient atmosphere. Re-enacting the Earth’s ecosystem may never be able to be completely replicated and it shows how special the Earth really is.
Most evolutionary ideas let life begin with single-celled water. But how did it come about?
Stanley Miller.
He conducted a well-known experiment. He produced a mixture of methane, ammonia, hydrogen and dihydrogen. And then he chased electricity through it for a week to recreate the lightning that ravaged the early oceans.
After a week of bubbling, organic compounds had formed. The soup suddenly contained sugars, fats and amino acids:
- Sugars contain energy.
- Fats are needed to make membranes.
- Amino acids form proteins and are the basic building materials for life.
The only thing missing are nuclear acids needed to build DNA.
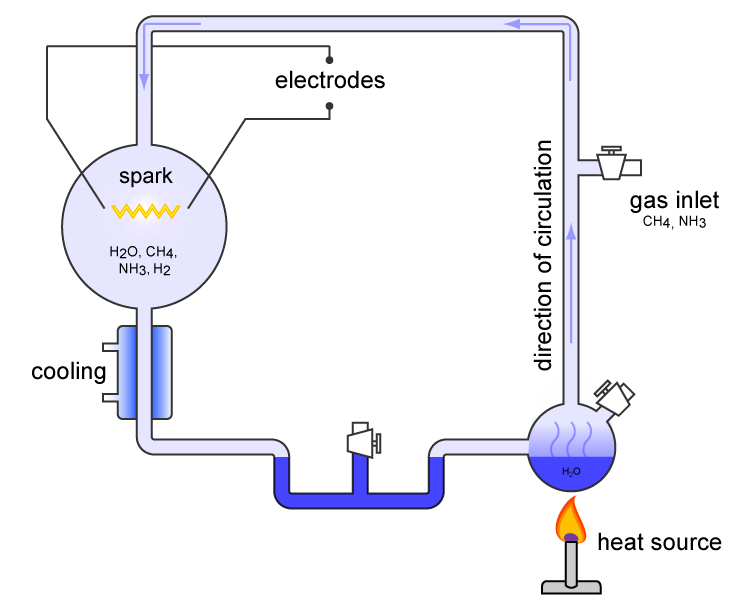
This illustration can be seen on the Wikipedia website.
Carbon.
All life on Earth is based on carbon. This allows millions of types of organic molecules to be combined, such as proteins and amino acids. All these building materials or polymers consist of a handful of the same atoms: so carbon, but also hydrogen, oxygen, nitrogen, phosphorus and sulfur.
But those same carbon molecules are vulnerable on a proto-Earth. Yet somewhere on that early Earth there must have been a place where the conditions were such that the first life could arise from those essential components. So, from the very first moment, there must be mechanisms that protect the beginning of life.
In Australia, remnants of stromatolites have been found deep underground. These are clumping carbon-nuggling microorganisms from 3.5 billion years old. For now, those are the germs of life. Apparently, these organisms seemed to be saving themselves with the energy from the earth’s interior.
Silicon.
On Earth, silicon is 1000 times more common than carbon. However, our biochemistry is entirely carbon-oriented.
However, life is thought to be possible on the basis of silicon. But the number of molecules that can be made with them is very limited:
- Silicon can only form 55 molecules with hydrogen and some other atoms. A further combination of these 55 molecules into larger polymers is very difficult and moreover, they are usually insoluble in water.
- This is not the case with the 29 000 possible molecules that are based on carbon. Moreover, it is possible to combine easily with these 29 000 molecules and are practically all soluble in water. So this is a serious threshold to keep silicon from being the backbone of life.
Silicon is in the Table of Mendelyev in the same column as carbon. It is heavier and less active than carbon even though there are as many binding places. At higher temperatures, silicon would behave more stablely than carbon. This could then give an advantage in some places in the Milky Way. The fact remains that a silicon-based life is not compatible with our earthly life. We might not even be able to detect it.
And with that, we’re back to carbon. Life elsewhere in the universe should logically also be based on carbon compounds. That’s what astrobiologists are still looking for.
DNA.
All life, including the simplest form of life, consists of information recorded in large molecules such as the DNA:
- This fragile and complex DNA is necessary to send numerous processes into the cell.
- In addition, DNA has the ability to use that stored information and pass it on to posterity. This replication becomes very tangible when we look at reproduction (life).
How such information and replication can arise by chance is unknown. There is no known law of physics that can produce information from nothing.
Chemical miracle.
The cell may be a simple object in biology, but it is a miracle of complexity:
- She can produce a fully working copy of herself within twenty minutes, a modern computer can do a lot, but we are still very far from the point that it could copy itself.
- Through her metabolism, she can capture energy to power all the necessary processes. In this metabolism, proteins play a crucial role. Proteins consist of amino acids. In addition, the question arises whether amino acids can arise spontaneously?
- Essential and also very banal is the membrane that envelops the cell and thus holds things together. This membrane is super efficient in its simplicity. Each membrane consists of phospholipids. Again the question whether phospholipids can arise spontaneously?
The possibility of such a cell spontaneously forming by chance defies any realistic probability. It is therefore an extremely difficult scientific problem.
Goldilocks.
A planet must have many special properties to make life possible. Everything has to be right from start to finish or all the dominoes fall over.
Life on earth has taken an erratic course, coincidences became the norm. It’s changed the planet. Then and now can’t be turned around.
Covered: atmosphere, magnetic field, rotation, sun, moon, minerals:
- Atmosphere:
- There must be sufficient attraction to maintain an atmosphere.
- But the atmosphere should not cause such strong pressure that it crushes everything on the planet.
- It seems that the mix of about 20 percent oxygen and 80 percent nitrogen is ideal for life as we know it. Life has caused this mix itself.
- Magnetic field:
- A key factor that protects a planet from the destructive effects of its star is a magnetic field. The Earth’s magnetic field stops the flow of electrons and protons from the sun so that it does not touch the Earth.
- That magnetic field should not be too strong or too weak. If it were too strong, it would tear apart the planet’s crust. Too weak a field does not stop enough particles from the parent star.
- Rotation:
- Another condition for life is the speed at which a planet orbits its axis.
- A planet that rotates completely every hour gives living organisms too little time for rest and activity.
- At a rotational speed of once a month, the days and nights are far too long.
- Sun:
- After all, life is highly dependent on liquid water. Most life occurs in places where the temperature varies somewhere between the freezing point and the boiling point of water.
- If the average distance from the sun was 5 %, all the water on earth would freeze. If the Earth were 5% closer to the sun, the earth would turn into an unlivable sauna. Note that the 5% of the margin is very small.
- The sun must be of an average age and be of a certain type. It has been shining at a steady pace for several billion years, thus providing a constant supply of energy to our earth. She has a predictable ending, but that is infinitely far from our bed, so it may leave us indifferent.
- Moon:
- The moon is no less important for life on Earth. It has roughly one-sixth of earth’s gravitational pull. This prevents the moon from swinging back and forth. In that case, no seasons would be possible.
- Due to its gravity, the moon causes tides on Earth Through the tides the water of the oceans mixes at different depths. In this way, the oceans become homogeneous and buffer the temperature fluctuations:
- A moon that was too small would greatly impoverish life on Earth because there would be hardly any ebb and flow. An important biotope could be lost.
- With an oversized moon, the floodwaters would wash over the continents and cause a huge erosion. The earth would become unlivable because of this loss of habitat.
- Minerals:
- One knows on Mars only 500 kinds of minerals and on Venus 1000. On Earth there are more than 4600.
- Two thirds of these minerals are directly or indirectly formed by life itself.
- That life has so well foreseen itself can seem suspicious. With one stroke of the pen, the supernatural lurks.
- Or should we accept time and chance, the two legs on which the theory of evolution rests?




Comments